Sleep Apnea Medication in 2026: What’s Real and What’s Coming Next
If you’ve been told you have obstructive sleep apnea (OSA), you’ve probably heard the same “big three” treatments: CPAP, weight loss, and sometimes a dental device or surgery.
For years, people asked one question:
“Is there a medication for sleep apnea?”
Now, that answer has changed.
The latest trend is not a “sleep apnea sleeping pill.” It’s treating the biology that drives OSA—especially obesity-related OSA, and the brain–airway control problem that makes your throat collapse at night.
This post is a deep dive into what’s new, what the research shows, and what to watch next.
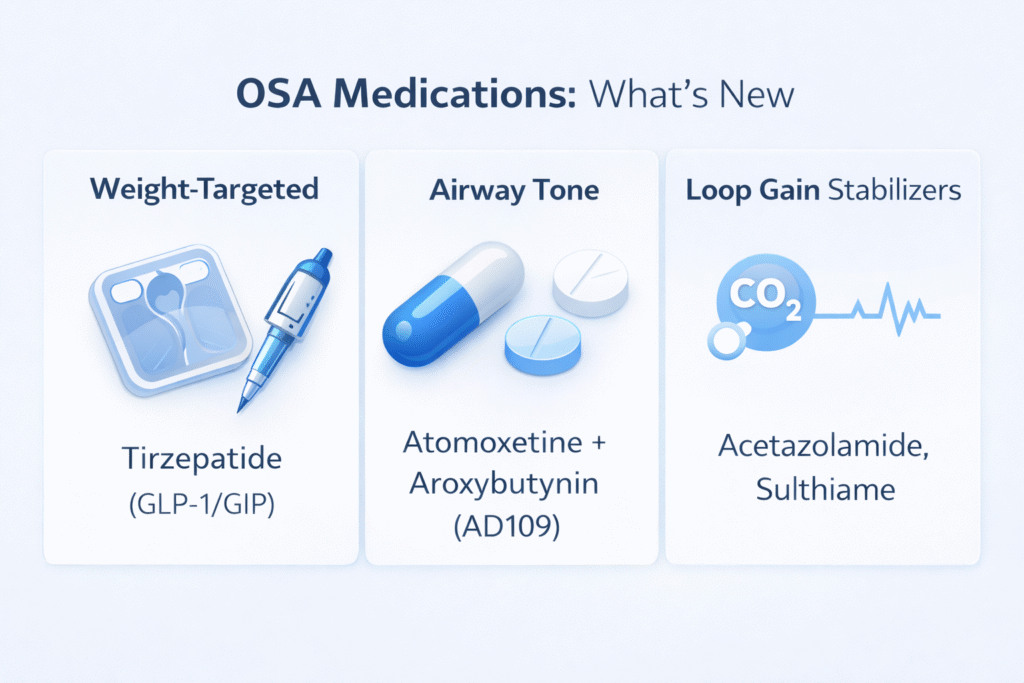
Quick update: What is the newest approved medication for obstructive sleep apnea?
In the U.S., the FDA approved tirzepatide (Zepbound) on December 20, 2024 for moderate-to-severe OSA in adults with obesity, used together with diet and physical activity.
This is a major milestone because it is the first FDA-approved medication specifically for OSA (for that subgroup).
Why OSA finally has “real” drug therapy now
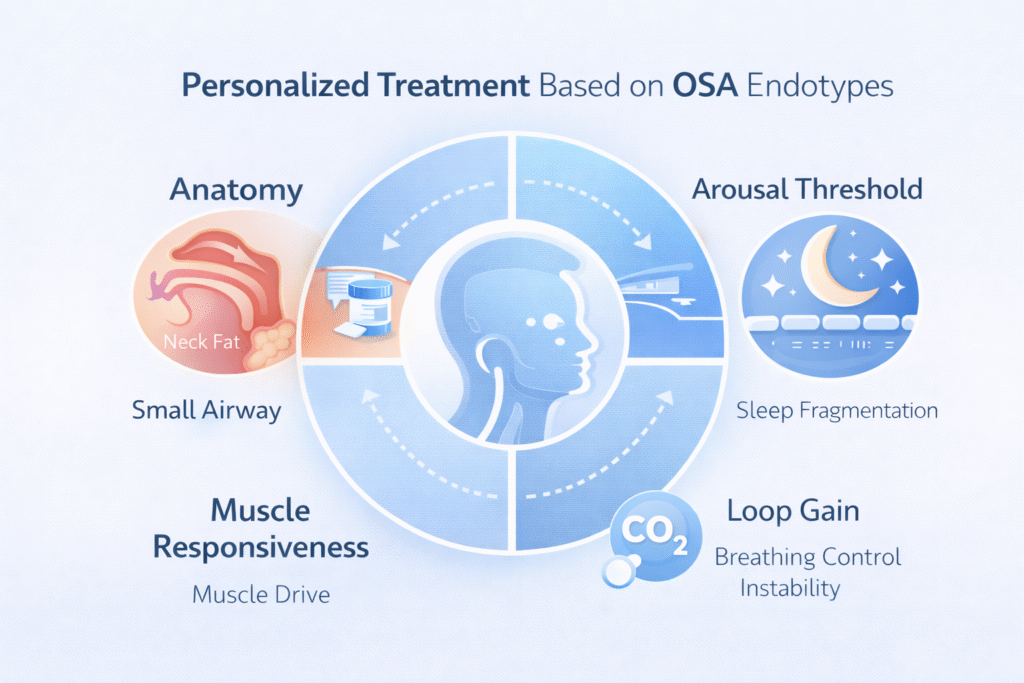
OSA is not one single disease. It’s a syndrome with different drivers:
- Anatomy: a narrow airway that collapses.
- Muscle control: airway muscles that don’t respond strongly enough during sleep.
- Breathing control instability (“loop gain”): the brain overcorrects breathing and triggers more collapse.
- Arousal threshold: waking too easily can fragment sleep and worsen instability.
For a long time, we had devices and procedures. Medications struggled because they targeted one piece of a multi-pieceproblem.
The new wave of OSA medications follows two big ideas:
- Treat the biggest upstream driver (weight/adiposity) in the right patients.
- Match medication to the patient’s OSA “type” (endotype/phenotype).
Trend #1 (the biggest one): GLP-1/GIP medicines for obesity-related OSA
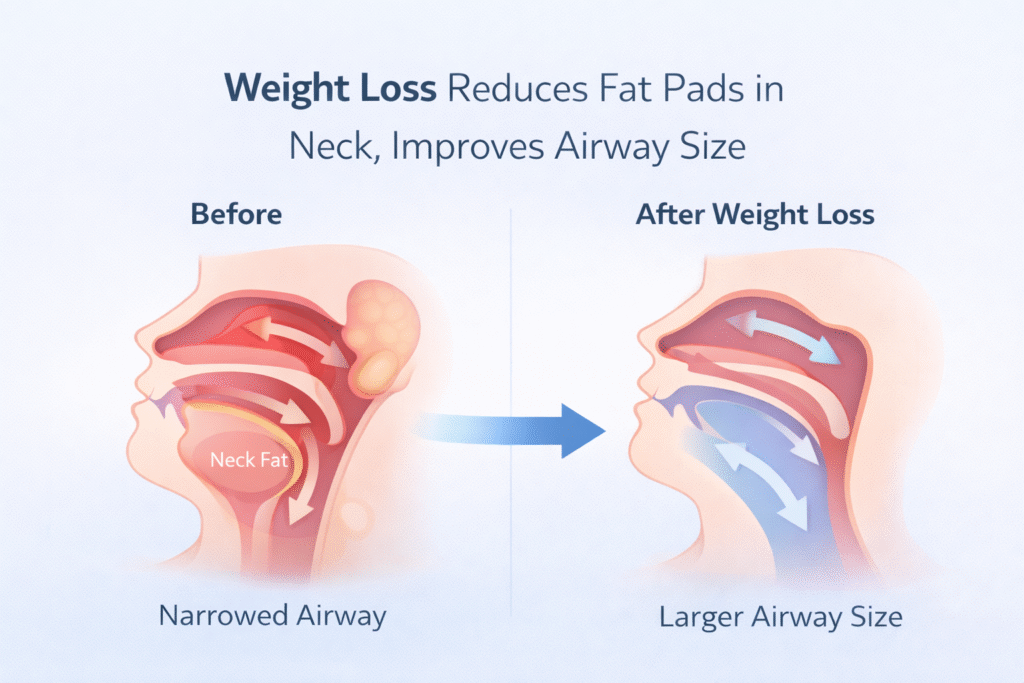
The headline
Tirzepatide (a dual GIP/GLP-1 receptor agonist) reduced OSA severity in large phase 3 trials (SURMOUNT-OSA).
What the research actually showed
In the NEJM SURMOUNT-OSA trials (52 weeks), participants had severe OSA at baseline (mean AHI ~50/hour).
- Trial 1 (not using PAP): mean AHI change at week 52 was −25.3/hour with tirzepatide vs −5.3/hour with placebo (estimated difference −20/hour, p<0.001).
- Trial 2 (on PAP): mean AHI change at week 52 was −29.3/hour with tirzepatide vs −5.5/hour with placebo (estimated difference −23.8/hour, p<0.001).
That’s not a “small improvement.” That’s a shift many patients would feel.
Who is it actually for?
Based on the FDA indication, this is for:
- Adults with obesity
- Moderate-to-severe OSA
- Used with diet + physical activity
Important reality check
- This is not “a replacement for CPAP” for everyone.
- Some people will still need CPAP, a mandibular advancement device, positional therapy, surgery, or a combination.
- Regulatory approvals differ by country (so what’s approved in the U.S. may not be approved where you live yet).
If you want a simple way to think about it:
CPAP splints the airway open tonight.
Anti-obesity medication changes the terrain over months.
Both can matter.
Trend #2: A true “OSA pill” that targets airway muscle tone (AD109)
This is the other exciting direction: instead of treating weight, treat the night-time airway collapse reflex.
The concept
OSA often worsens because upper-airway muscles relax in sleep. One strategy is to:
- Increase upper-airway muscle drive (via noradrenergic pathways)
- Reduce unwanted anticholinergic effects that can destabilize sleep or physiology
- Improve airway stability without a mask
The medication in the spotlight: AD109 (investigational)
AD109 is a fixed-dose combination (aroxybutynin + atomoxetine) being studied as an oral therapy for OSA. Phase 3 trials (LunAIRo and SynAIRgy) were designed to confirm longer-term efficacy and safety.
Recent topline Phase 3 announcements reported significant AHI reductions vs placebo at 26 weeks, with continued effect reported at longer follow-up, and improvements in oxygenation metrics.
What about earlier peer-reviewed trials?
This class of approach has been building for years.
- A landmark randomized crossover trial showed that atomoxetine + oxybutynin can greatly reduce OSA severity.
- In a later randomized study (AD036), AHI fell from a median of 14.2/hour on placebo to 6.2/hour with atomoxetine+oxybutynin (and even lower with atomoxetine alone in that study), with improvements in oxygen metrics.
- A Phase 2 trial of AD109 also reported efficacy and safety over 4 weeks.
Where this is heading
This “airway muscle tone pill” approach is part of a bigger trend: endotype-driven treatment—choosing medication based on what is driving your OSA.
Trend #3: Lowering “loop gain” with carbonic anhydrase inhibitors (acetazolamide, sulthiame)
Some people with OSA have unstable breathing control. Their system “overreacts,” creating a cycle that promotes apneas/hypopneas.
Carbonic anhydrase inhibitors can stabilize ventilatory control. A 2025 Lancet review notes acetazolamide reduced AHI by about ~38% in a meta-analysis (dose-dependent in many studies).
Sulthiame (investigational) is getting attention
Sulthiame has been studied for OSA severity reduction and for how it modifies OSA endotypic traits (ventilatory stability and airway factors).
A Lancet publication/coverage around 2025 also highlights sulthiame as a potential option in this mechanism-based category.
What medication is
not
treating the obstruction (but still matters)
You may see medicines prescribed for:
- Excessive daytime sleepiness (even when OSA is treated)
- Alertness, driving safety, work functioning
These can be helpful in selected cases, but they do not fix the collapsing airway. They treat a consequence, not the core mechanism.
So… are we entering a “no-CPAP future”?
Not exactly.
What we are entering is a combination era:
- Some patients will do best with CPAP + weight-loss medication (especially obesity-related OSA).
- Some may eventually use an oral OSA drug if trials translate into approvals and real-world safety/response holds up.
- Others will still need mandibular advancement, positional therapy, nasal optimisation, or targeted surgery.
The direction is clear: OSA treatment is becoming personalised.
FAQ
Is there a pill for obstructive sleep apnea?
In the U.S., tirzepatide (Zepbound) is FDA-approved for moderate-to-severe OSA in adults with obesity (with lifestyle changes).
Other “OSA pills” (like AD109 or sulthiame) are still investigational as of the latest public reports.
Will Zepbound cure sleep apnea?
It can significantly reduce severity in many patients with obesity-related OSA, but “cure” depends on your anatomy, baseline severity, and other drivers. In trials, AHI reductions were large on average, but responses vary.
If I lose weight with medication, can I stop CPAP?
Sometimes—but don’t guess. The safe way is a repeat sleep study (home sleep test or lab PSG) after weight stabilises.
What’s the next big medication coming for OSA?
The most watched pipeline areas are:
- Oral neuromuscular agents (like AD109)
- Ventilatory control stabilisers (carbonic anhydrase inhibitors like sulthiame/acetazolamide approaches)
References
- U.S. Food and Drug Administration. FDA Approves First Medication for Obstructive Sleep Apnea. FDA News Release. December 20, 2024. Accessed January 5, 2026.
- Malhotra A, Grunstein RR, Fietze I, et al; SURMOUNT-OSA Investigators. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N Engl J Med. 2024;391:1193–1205. doi:10.1056/NEJMoa2404881.
- Schmickl CN, Malhotra A. Drug therapy for obstructive sleep apnoea: promises and challenges. Lancet. 2025 Oct 9;406(10514):1928–1930. doi:10.1016/S0140-6736(25)01379-0.
- Taranto-Montemurro L, Messineo L, Sands SA, et al. The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity: a randomized, placebo-controlled, double-blind crossover trial. Am J Respir Crit Care Med. 2019 May 15;199(10):1267–1276.
- Schweitzer PK, Maynard JP, Wylie PE, Emsellem HA, Sands SA. Efficacy of atomoxetine plus oxybutynin in the treatment of obstructive sleep apnea with moderate pharyngeal collapsibility. Sleep Breath. 2023 May;27(2):495–503. doi:10.1007/s11325-022-02634-x.
- Taranto-Montemurro L, Patel SR, Strollo PJ Jr, et al. Aroxybutynin and atomoxetine (AD109) for the treatment of obstructive sleep apnea: rationale, design and baseline characteristics of the phase 3 clinical trials. Contemp Clin Trials Commun. 2025 Aug 17;47:101538. doi:10.1016/j.conctc.2025.101538.
- Apnimed, Inc. Apnimed Announces Positive Topline Results in the First Landmark Phase 3 Clinical Trial of AD109. News release. May 19, 2025.
- Apnimed, Inc. Apnimed Reports Positive Topline Results from Second Phase 3 Trial of AD109. News release. July 23, 2025.
- American Academy of Sleep Medicine. Apnimed announces positive results from second clinical trial of sleep apnea medication. Industry news. July 23, 2025.
- Schmickl CN, Landry SA, Orr JE, et al. Acetazolamide for OSA and central sleep apnea: a comprehensive systematic review and meta-analysis. Chest. 2020;158(6):2632–2645. doi:10.1016/j.chest.2020.06.078.
- Ni Y-N, Yang H, Thomas RJ. The role of acetazolamide in sleep apnea at sea level: a systematic review and meta-analysis. J Clin Sleep Med. 2021 Jun 1;17(6):1295–1304. doi:10.5664/jcsm.9116.
- Randerath WJ, Grote L, Stenlöf K, et al; FLOW study investigators. Sultiame once per day in obstructive sleep apnoea (FLOW): a multicentre, randomised, double-blind, placebo-controlled, dose-finding, phase 2 trial. Lancet. 2025;406(10514):1983–1992. doi: 10.1016/S0140-6736(25)01196-1.

Comments are closed